Abstract
Introduction Clinical trials suggest that the burden of Acute Myeloid Leukemia (AML) to patients is substantial, with commonly observed comorbidities associated with disease and treatment including infections, hematologic, hemorrhagic, cardio/cerebrovascular, hepatic, and renal events. There are no large population-based real-world studies that assessed comorbidities and complications in AML patients overall and in particular, among the most challenging AML patients to treat: relapsed and/or refractory (R/R) patients.
Objective To estimate frequency, incidence rates, and time to event for selected incident comorbidities and complications among AML patients and a subgroup with evidence of R/R disease, compared to non-cancer patients, leveraging a large adjudicated claims database.
Methods A retrospective cohort analysis was conducted using the QuintilesIMS Real-World Data Adjudicated Claims - US (formerly known as PharMetrics Plus) database covering the years 2005-2015. Adult patients (>18 years) with at least one inpatient or outpatient claim indicating a diagnosis of AML (ICD-9-CM code: 205.0x) between January 1, 2006 and October 31, 2015 were identified, with index date defined as the date of the first observed AML diagnosis. A comparison patient of the same age, gender, geographic region, insurance type, and patient enrollment year without evidence of any cancer during baseline was matched 1:1 to each AML patient and assigned the same index date as the respective AML match. All patients were followed from 12 months prior to index (baseline) through 12/31/2015, disenrollment from the health plan, or death, whichever came first (with requirements of a minimum 2 months follow-up). Patients were considered to have evidence of refractory AML if they had >2 cycles of chemotherapy (new cycle defined as 14-60 days since previous chemotherapy; same cycle if <14 days from previous chemotherapy). Patients were considered to have evidence of relapsed AML if they had at least one diagnosis code indicating relapse (ICD-9 205.02), or received chemotherapy after a >60-day period with no evidence of chemotherapy, or diagnosis/procedure codes indicating repeated stem cell/bone marrow transplant. Study measures included frequency, incidence, and time to event for infections, hematologic, cardio/cerebrovascular, hepatic, hemorrhagic, renal events, and other events of interest during follow-up. Separate Multivariate Cox Proportional Hazards models were used to evaluate associations between AML and three specific outcomes (cardiovascular, type 2 diabetes [T2D], stroke) in the full study sample, controlling for baseline demographic (age, sex, region) and clinical characteristics (NCI comorbidity score, history of myelodysplastic syndrome).
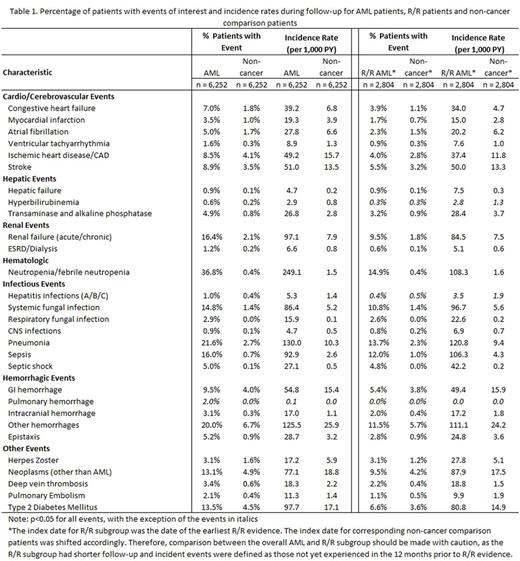
Results A total of 6,252 AML patients were identified and matched 1:1 to non-cancer patients (mean age: 54 years, 54% male in both groups). AML patients had higher NCI comorbidity index scores during baseline than non-cancer patients (0.9 vs. 0.3, p<0.001). These patients had higher incidence rates of selected comorbidities and complications during follow-up and shorter times to events than non-cancer patients (Table 1). The most common comorbidities and complications were neutropenia/febrile neutropenia (36.8%, 249.1 per 1,000 person-years [PYs]), pneumonia (21.6%, 130.3 per 1,000 PYs), and other hemorrhages (20.0%, 125.5 per 1,000 PYs). Results from Cox models suggest that AML patients had significantly higher risks of cardiovascular events (HR=3.9, 95% CI 3.4-4.6), T2D (HR=4.7, 95% CI 4.0-5.5), and stroke (HR= 2.6, 95% CI 2.2-3.1), all p<0.01. A subset of 2,804 study patients had evidence of R/R AML, including 1,225 (19.6%) with refractory (mean ± SD time to refractory disease 3.7 ± 2.6 months) and 1,579 (26.3%) with relapse (mean ± SD time to relapse 4.2 ± 7.4 months). Results were similar in terms of patterns of incident comorbidities and complications for the R/R subgroup.
Conclusions This retrospective analysis found that AML patients had higher baseline comorbidities and higher rates of selected incident comorbidities and complications after diagnosis compared to non-cancer patients. The results were similar in R/R patients. This study provides real-world evidence of overall pre-disposition and risks among AML patients that might have clinical significance in terms of treatment, patient management, and risk communication.
Near: QuintilesIMS: Employment; Daiichi Sankyo Inc.: Research Funding. Iqbal: Daiichi Sankyo Inc.: Employment. Huang: QuintilesIMS: Employment; Daiichi Sankyo Inc.: Research Funding. Dhopeshwarkar: Daiichi Sankyo Inc.: Employment. Lang: QuintilesIMS: Employment; Daiichi Sankyo Inc.: Research Funding. Wallis: Daiichi Sankyo Inc.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.